Salt in Marine Boilers
This page is adapted from Henry Evers, LL.D., Steam and the Steam Engine : land and marine, London, William Collins, Sons, and Company, 1873. Chapter X, pp161-175. The author was Professor of Mathematics and Applied Science at the Charles Science School, Plymouth.
Notes: Page numbering is inserted e.g. [page 162]. Images have been placed with relevant text, and may appear higher in the original text layout.
Also available in PDF format with the original layout, including the "Exercises" at the end of the Chapter.
CHAPTER X.
SALT IN MARINE BOILERS.
Sea Water – Specific Gravity – Boiling Point – Blowing Out – Scale – Salinometer – Hydrometer – Priming – Feed Pumps – Giffard's Injector
183. Pure Water Should be Used. –Boilers, both land and marine, are liable to become internally incrusted. If these incrustations are not carefully removed or guarded against great injury will ensue. All water contains solid substances, whether it be lime, flint, salt, or sulphur, all of which will either do, or be the means of causing, damage. Marine boilers are generally fed with salt water. Hence it is necessary to explain fully the constituents of sea water, and how their evil effects may be guarded against.
The deposits and incrustations which are the source of so much danger, are not likely to be retained as necessary evils. If surface condensation, which, as we have already said, has been introduced into some of our iron-clad vessels, be successful, the condensed water, being free from all such matters, will form no deposits. If the steam could be rapidly and effectually condensed without mixing it with impure water, it would itself supply almost enough water for feed, and that of the purest quality. All the evils of deposits, incrustations, priming from impure water, and much of the wear and tear of boilers, would be in many cases entirely and others greatly prevented. The consumption of fuel would be less than at present, and the air pump would be considerably reduced in size, and therefore less power would be required to work it, although of course we should have the circulating pumps instead, but still upon the whole there would be a gain. As the condensed steam would contain no air, the [page 162] function of the air pump would be exclusively confined to the removal of the condensed steam.
184. Sea Water is both salt and bitter. Everywhere the sea holds in solution a large quantity of solid substances, chiefly common salt or chloride of sodium. The amount of salt is not constant in all seas, nor even in the same sea, nor at all depths, varying according to the amount of evaporation (i.e., the heat of the climate) and the quantity of river water running into the sea. The Red Sea is Salter than the Mediterranean, the Mediterranean than the Atlantic, the Atlantic than the Pacific. The water of the northern hemisphere is not so salt as that of the southern. The position of maximum saltness in the ocean is about 22° N. latitude and 17° S. latitude, and the belt of ocean lying between. We may incidentally mention that this is the region of greatest evaporation, and that therefore the saltness of the ocean follows from that circumstance. The Polar seas, Baltic, and White seas, contain very little salt. Ice is free from it, because water in the act of freezing parts with all its impurities. Out of every 1000 parts 34.4, or about 1/30 of the whole consists of solid matter; out of the 34 parts nearly 24 are common salt. We may put it thus : out of 30 gallons of sea water, 1 gallon consists of solid matter, and of this solid matter 24/34 or 12/17 is pure salt; 24 parts out of 34.4 are pure salt, 4 parts chloride of magnesium, 4 parts sulphate of soda; 1 part in 1000 is carbonate of lime (chalk), and 1 part in 4000 silica (flint).
ANALYSIS OP SEA WATER.
| Chloride of Sodium | 24 |
| Chloride of Magnesium | 4 |
| Sulphate of Soda | 4 |
| Carbonate of Lime | 0.34 |
| Silica | 0.080 |
| Other substances * | 2 |
| Total | 34.426 |
* Bromine, Iodine, Boron, Silver, Copper, Iron, Potassium, etc.
[Page 163] Professor Forehammer [See Ansted's Physical Geography, p. 141.] gives the following as his analysis of sea water:
| Chlorine | 19 parts |
| Sulphuric Acid | 2.26 parts |
| Lime | 0.56 parts |
| Magnesia | 2.10 parts |
| All salts | 34.04 parts |
| Total parts | 58.32 |
Carbonic acid gas is ever present in sea water, and its quantity increases with the depth. There is also a trace of ammonia with atmospheric air to sustain life in the proportion of from 1/40 to 1/30 of its bulk. These facts, especially that relating to the different quantity of salt in different seas, go to explain the reason why the extent of the "brining" varies in different seas.
185. The Specific Gravity of sea water differs with every sea. In the North Atlantic Ocean it is about 1.02664, while in the South Atlantic it is greater, 1.02672. The Indian Ocean has a specific gravity of 1.0263; the Red Sea, 1.0286; the Mediterranean, 1.0289.
186. Boiling Point of Sea Water. – In consequence of some of the above solid substances being chemically combined and the others mechanically suspended in sea water, especially because of the latter, and its specific gravity being greater, it takes considerably more heat to boil it than to boil fresh, spring, or river water, and of course as ebullition continues and the steam is used the water will get salter and salter; no salt can possibly pass away with the steam, and therefore the amount of heat required to convert the water into steam will have to be increased in proportion to the density of the water, while the water itself will become saturated with salt, or it will be incapable of holding more salt, which will be precipitated, and form a crust on the boiler, separating the iron boiler plates from the water, so that the boiler plates can actually become red hot and danger is imminent, for the plates being softened they are liable to collapse.
187. Boiling Point of Salt Water. – Salt water containing 1/30 part of salt (it has been usual in all works on steam to say 1/35) will boil at a temperature of 100°2/3 C.; if the proportion, of salt be doubled, or 2/30, it will boil at a temperature of 101°1/3 C; if 3/30 or 4/30 the boiling point will rise respectively to 102° C. and 102°2/3 C.; when there are 12/30 of salt in the water the boiling point rises to 107°7/9 C. 12/30 is the point of saturation, when the water is so full of salt that it will hold no more, and it is therefore rapidly precipitated. It will assist the memory perhaps to state that in each gallon of sea water there is more than four ounces of salt, and if two gallons be boiled down to one, it will contain double that amount, or more than eight ounces.
188. Blowing Out or Brining the Boilers. – Generally the saltness of water in the boilers must be kept below three or four thirtieths. To effect this, and to have them as free from salt as is consistent with the economical consumption of heat, the practice of "blowing out" is resorted to. For this purpose blow out cocks are fitted to the bottoms of all marine boilers, from the cocks pipes lead into the sea. Every two hours, but generally less, the blow out cocks are opened, and the supersalted water violently forced out of the boiler, by the pressure of the steam, into the sea. Much heat is lost by this blowing out, and many methods have been devised to save it. Before showing how this is accomplished, we must give other modes of getting rid of the impurities which collect in a marine boiler. The brine is sent overboard,
(1) By Blow Out Cocks (already explained).
(2) By Brine Pumps.
(3) By Surface Blow Out and Scum Cocks.
189. (2) By Brine Pumps. – To many engines are fitted brine pumps, and at every revolution of the engine a small portion of brine is extracted from the boiler. The size of the brine pumps must be such that the quantity of water drawn off added to that evaporated must be equal to the quantity introduced by the feed pump. If the water ejected from the boiler is to contain 3/30 of salt, or three times as much as the feed water, then, if the feed pump supply n gallons in a given time, the brine pumps must extract n/3 gallons in the same time. The rule is, blow out from 1/4 to 1/3 the amount of feed water.
[page 165] 190. (3) Surface Blow Out and Scum Cocks. – The foreign substances in a boiler are always buoyed up to the surface, where they not alone prevent ebullition, but the formation of steam. The steam rises from and around them, and they remain at the surface for some time, when they gradually descend and form a scale upon the tubes and flues. It is therefore found quite as advantageous to blow out from the surface as from the bottom of the water. It is done by means of scum cocks, which are inserted on a level with the water, and are kept constantly about one-eighth open the whole of the time, so that as fast as dirty scum and other impurities rise to the surface they are expelled.
191. Lamb's Surface Blow Out Apparatus is a very efficient contrivance for effecting the same object. A float in connection with the bottom of the discharge pipe regulates the feed and discharge water. The apparatus ejects the scum and dirt at once; but in some boilers sediment collectors are employed, one, in shape and size somewhat resembling a sugar loaf, is placed in each boiler with the small end or apex downwards, it is connected to a pipe leading into the sea to carry the sediment away. The top or base of the cone stands out of the water, and the impurities enter through longitudinal tapering slits being ballooned into the cone, where the water is comparatively still, by the steam as it rises to the surface. The object of all this is to save heat.
192. Scale. – Whatever care and precaution are adopted, scale can hardly be prevented from forming on the boiler plates. A careful and attentive engineer can always reduce it to a minimum. When scale is formed on the boiler plates, it prevents the passage of heat into the water, for salt, gypsum, lime, etc., are exceedingly bad conductors of heat, and will not allow its motion to pass to the water, and therefore a waste of fuel must arise. When water is saturated with salt, etc., through negligence or otherwise, it becomes heavier, and therefore takes more heat to boil it, which is another waste of fuel; again, the scale is occasionally so hard and solid that the plates become red hot, and are liable to be burnt as well as to give way from internal pressure. Ammonic chloride and other chemical substances are sometimes put into marine boilers to prevent scale, but [page 164] the utmost they do is to precipitate the foreign ingredients as powder, which must still be removed by blowing out. The more of these substances there are in the water, the more work the heat has to do to lift them, and therefore the more heat is required for ebullition, which is waste of motion and power.
A practical engineer, who has examined thousands of boilers, says : "Much mischief is often done by the injudicious use of compositions in the boiler which are designed to prevent incrustations, especially where there is no blow off cock or where its use is neglected. A hard deposit on the boiler plates is, in the writer's opinion, not so injurious as the soft and muddy deposit produced by the use of such compositions. A hard scale ... is sufficiently mischievous, but the injury to the plates is much more rapid when a thicker but spongy deposit entirely prevents contact of the water, and impedes the transmission of the heat. The money spent in boiler compositions would be better applied in securing a supply of proper water, or in filtering and purifying the water before it enters the boiler. More attention to the purity of feed water would nearly always effect economy, and would be far cheaper than using chemical or other ingredients to neutralize the impurity after it is in the boiler. In many cases simply filtering the water in some ready way has produced very great improvement." [* from Marten's SteamBoiler Explosions.]
A simple illustration of the formation of scale may be seen by examining the tea-kettle, where a scale (lime or chalk chiefly) is left on the sides and bottom of the kettle, because steam formed from impure water is perfectly pure; it can carry nothing away with it. We may also consider the boiler as, or compare it to, a great salt-pan. Just as in Cheshire and Worcestershire salt is made by the simple process of evaporating water in large pans, so does salt, etc., collect in marine boilers; but there is this difference, the scale formed on boilers is not soluble in water, while salt is. Here, of course, we draw a distinction between salt and scale.
An effective and expeditious, but not very good plan, to scale boilers is to throw in a few wood shavings [page 167] all along the bottom, and set them on fire. They quickly heat the scale, which expands more than the shell of the boiler; the heat cannot reach the latter, so the scale is loosened from the plates. Precisely the same process is gone through, with a different result, when a glass tumbler is cracked by pouring hot water into it. The heat in the water suddenly expands the inside of the glass, which becomes too large for the outside, and so the glass is broken. Any scale that remains after this must be taken off with a hammer and chisel. This hard incrustation is formed in layers, and of course chiefly consists of carbonate and sulphate of lime, gypsum and chalk, with common salt. We have by us pieces of scale looking like pieces of iron; in their cross section they have the appearance of very thin alternate bands of iron and hard crystalline rock, while other pieces are pure salt. On this point Mr. Marten says : "The practice, especially in certain districts, of emptying the boilers immediately the engines are stopped, and before the flues have cooled, in order to loosen the scale by overheating the plates, has caused much more mischief than those who persist in doing it will believe, and has nearly ruined some otherwise good boilers."
193. Salt and the Boiling Point. – There are several methods of ascertaining the amount of saturation of the water in a marine boiler :
(1) By the Thermometer.
(2) By the Hydrometer.
(3) By the Salinometer.
From what has been said it will be gathered that the boiling point of water depends upon the quantity of salt in it, its specific gravity, and the pressure of the air. The strength of a solution of salt and water has always a fixed and well ascertained relation to the boiling point and specific gravity.
For water with
1/30 or 1° of saltness in it boils at 100°2/3C.
2/30 or 2° " " l0l°1/3 C.
3/30 or 3° " " 102° C.
4/30 or 4° " " 102°2/3 C.
5/30 or 5° " " 103°1/3 C.
10/30 or 10° " " 106°4/9 C.
12/30 or l2° " " 107°7/9 C.
[page 166] And also as fresh water when the barometer stands at
27 inches boils at a temperature of 97°.2C.
28 " " 98°.1 C.
29 " " 99°.1 C.
30 " " 100° C.
31 " " 100°.8 C.
we see at once the truth of what was previously said, that the boiling point of water depends upon its weight or specific gravity and the pressure of the air.
If, then, water be taken from the boiler, and boiled in the engine room under the ordinary barometric pressure of the air, and it is found by using the thermometer that its temperature at the boiling point is 103°23/9, we must at once conclude that there are 5 degrees of saltness in the water, and that precipitation of impurities is commencing, and blowing out must be resorted to at once. But if by the same process it is ascertained that the water boils at 101°1/3 C. (in the engine room), it is known that the boiler is comparatively safe and in good working condition. Salt does not really deposit till 12/30.
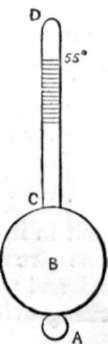
HYDROMETER
194. The Hydrometer tells us the amount of salt in water by showing its specific gravity. The figure in the margin represents one. B is a hollow ball of brass or other metal, from which rises a stem C D, graduated; A is a second globe filled with mercury to make the whole swim uprightly in the water. A acts in precisely the same manner as the lead on a fishing line. The lead keeps the float upright, so does A the hydrometer. The stem C D is graduated that we may read off how far the stem sinks in the water. The greater the specific gravity of the water, or the more salt there is in it, the less it will sink, so the density is thus made a test to exhibit the amount of salt. We read off (not the density, but) the saltness of the water. Each hydrometer is graduated to a particular scale, generally 55°; i.e., when placed in distilled water at a temperature of 55° the hydrometer sinks to the point marked 55°. This is much too low, for when water is taken from the boiler the experimentalist has to wait a considerable time [page 169] for the water to cool down before he can test it. 90° C. would be a far better temperature to select. We now see the utility of the specific gravities of sea water given on page 163, and that the hydrometer is an imperfect instrument without the barometer; so useless is the one without the other, that we frequently see attempts made to combine the two, as in the salinometer.
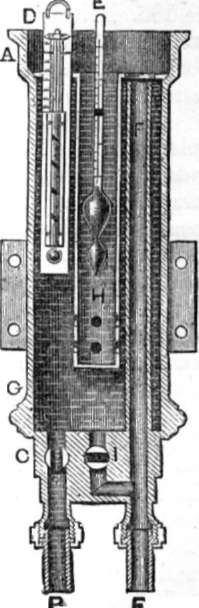
HOW'S SALINOMETER
195. Salinometer. – The salinometer has been presented in several shapes. In one it consists of a thermometer and hydrometer combined in a copper vessel, in another, Seaward's salinometer, of two pith balls. Mr. Seaward affixes a glass tube fourteen inches long, in a similar manner and in a corresponding place to the glass water gauge, so that when attached to the boiler the water rises up from the bottom of the boiler through the lower cock, and remains in the glass tube at the same level as the water in the boiler. The taps are then closed and the upper one opened, and two small balls of glass or metal are dropped into the water. The specific gravity of the first ball is such that it will sink when there are five degrees of saltness in the water and swim when more, the other ball will sink when there are less than three degrees of saltness, but swim when four or more. By this method the state of the boiler is soon ascertained.
How's salinometer consists of a cylindrical vessel, A G, connected with the steam boiler by the pipe B; the connection on the boiler being below the surface of the water. The quantity of water admitted to the salinometer is regulated by the cock C in pipe B. The salinometer is most usually fixed in the engine room, so as to be in constant view of the engineer, but it can be fixed in any other convenient place. A thermometer D is placed in the cylinder A G of the instrument, to show the temperature of the water. A hydrometer E [page 170] floats in the water, at a height corresponding to the density or saltness which it indicates, and is protected by the metal guard H. An overflow pipe F takes away the surplus water, and prevents it running over the top. I is a cock for emptying the instrument through the pipe F. It should, of course, be emptied as often as the water is tested.
198. Priming. – When the steam comes from the boiler mixed with water, in the shape of spray or froth, it is said to be primed. Priming exists under most diverse circumstances; its cause cannot at all times be clearly traced.
197. Causes and Danger of Priming. – Priming takes place more in new than in old boilers; when there is but little water in the boiler; when the spaces between the tubes and flues are contracted; when there is fierce ebullition, this cause may be said to accompany all priming; in passing from fresh water to salt or salt to fresh; when the water used is muddy, dirty, or slimy; when there is too small a steam chest; when a safety valve, being situated near the steam pipe, is suddenly opened. The danger arising from priming is very great, and should therefore be most anxiously guarded against. We shall see its danger and injurious effect, if we but consider that when it gets into the cylinder, and there accumulates as incompressible water, something must give way should the test cocks and escape valves act improperly. Priming impairs the vacuum; in consequence of this, more water will have to be used for condensation, which will throw a greater load upon the air pump, and more feed water will also be required.
198. Remedy for Priming. – As priming is generally accompanied with great ebullition, obviously the most effectual remedy will be to enlarge the steam chest. It is found that boilers with plenty of water surface, or with a large steam chest, seldom or never prime. Cornish boilers with their large water surface give no trouble by priming. A remedy much practised with locomotive boilers, is to open a safety valve remote from the steam chest and pipe. Other temporary remedies are : to partly shut the throttle valve; to work the steam at a high pressure; to open the furnace door, thus checking the fierce boiling; to put down the stop valve so that the steam rushes against it, and the water is knocked out; [page 171] to inject tallow into the boiler by means of the donkey pump or a syringe fitted on purpose, this is the favourite remedy, but it is found in some boilers to increase the priming. Another remedy is to fit a steam pipe in the boiler full of small holes, and inside this another similar pipe, but to take care that the perforations of one pipe are not opposite those of .the other. The steam in entering dashes against the inside pipe, and the spray falls out. Any thing that checks furious ebullition, or allows the steam plenty of space to rise, checks priming. When the steam chest has to be enlarged, it is better to fit a second on the top of the old one. Priming arising from the use of impure water may be obviated by liberally blowing off from the surface until the nuisance is abated.
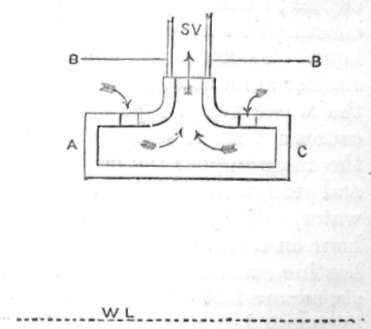
ENTRANCE TO STOP VALVE.
A very good plan to prevent priming is one adopted in the engines constructed by Charles Powis & Co. Their arrangement is to fit the stop valve, opening to boiler, with a disc plate, arranged with orifices on its upper side so that dry steam only can find its way through the stop valve. A C is a section of the disc plate fitted inside the boiler; W L is the water line, and B B the top of the boiler, so that all steam passing to the stop valve, which is situated just above S V, must pass in the direction of the arrows, through the small perforations into which the top arrows are entering. The water will be thrown and knocked out of the steam before it can pass to the stop valve.
Boilers sometimes prime when the ship passes from salt to fresh water or fresh water to salt. It has been suggested that in passing from salt to fresh water the cause is this : fresh water being lighter than salt, is upon its admission to the boiler more easily thrown about by the ebullition, and therefore more spray is flying; but as the same boiler will [page 172] also prime in passing from fresh to salt water, this reason evidently will not hold; we have yet to seek the true cause. May not the change of water cause a serious change in the existing condition of the boiler, and this change being accompanied by a general disturbance of the equilibrium of the water, much more spray is thrown off than usual, and priming follows. [* See Causes of Boiler Explosions – Spheroidal Condition of Water, and Water Purged from Air] When new boilers have primed, a good plan adopted, is to run into harbour and blow out the boiler several times in succession. This has often effectually prevented priming.
199. Fire Grate Surface, Heating Surface, Amount of Coal to Evaporate One Cubic Foot of Water. – In the majority of marine boilers, it is usual now to allow threequarters of a square foot of fire grate surface, and about nineteen square feet of heating surface, to each horse-power, but some take these numbers at half a square foot and twelve square feet. It is also calculated that six pounds of coals should be consumed every hour for each horse-power of the engine; these proportions of fire grate, heating surface, and consumption of coal, evaporate one cubic foot of water per hour. Locomotive boilers are constructed with a much smaller amount of fire grate surface; to compensate for this, the waste steam pipe is introduced into the funnel, which causes a most intense heat in the furnace, and it is found, the more intense the heat, or the hotter the heating surfaces and the water are, the more heat will pass into the water. They consume one hundred weight of coke per hour on each square foot of grate surface, the proportion of heating surface to this is eighty square feet; on every five or six square feet of heating surface one cubic foot of water is evaporated per hour. Each horse-power requires a cubic foot of evaporated water per hour, but in high pressure work more. The quantity of water may be generally taken as one cubic foot per horse-power per hour, but it is in excess for such engines as those in which advantage is taken of the expansive force of steam. In Cornish boilers, where an enormous duty is obtained for each engine, not more than three and a half or four pounds of coal is burnt on each [page 173] square foot of grate surface per hour. As well as a boiler having a due proportion of grate and heating surface to produce the necessary volume of steam, the furnace must be sufficiently roomy to consume all the products of combustion; the tube or flue surface, etc., must be adapted to abstract as large an amount of heat as possible, without too much passing away as waste, while at the same time the water spaces in the boiler and the distances between the tubes must be large enough to allow the steam freely to rise, or else priming may take place. Again, the furnaces should never be too long, for the stokers will find a difficulty in keeping the bars free from clinkers, the clinkers as well as the fire not being fairly within reach.
200. Feed Pumps. – The feed is supplied to the boilers in one of the following ways : (1) By boiler hand pumps; (2) by the donkey engine; (3) by the feed pump proper; or (4) by Giffard's injector.
(1) The boiler hand pumps are fitted to marine boilers, so that when there is no steam up men may fill the boiler by hand, providing it is not sufficiently below the level of the sea for sea water to run in freely when the Kingston valve is opened.
(2) The donkey is a small steam pump in the engineroom that can be set to work to fill up the boilers when the engines are waiting for orders. The donkey has always the steam piston and pump piston at opposite ends of the same rod.
(3) The feed pumps which have been already explained. In stationary engines part of the warm condensing water is driven into the boiler as feed; the rest, by far the greater, quantity, being allowed to run away. But the feed pumps should at all times be capable of supplying much more water than the boiler in its normal state will use. The capacity of the feed pump is generally about 1/240th that of the cylinder, so that it can supply more than three times as much as is required. While the steam pipe should be attached to the highest point of the steam chest, the feed pipe should be fixed as low down as possible, so that the cold water may gradually rise. In most Government vessels the feed and donkey pumps are made of brass.
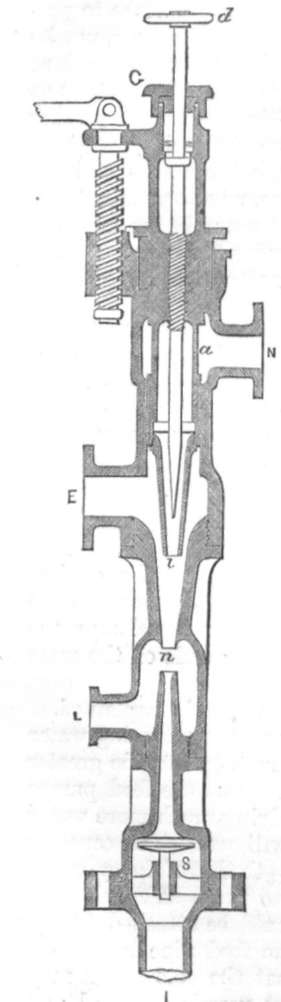
GIFFARD'S INJECTOR.
[page 174] 201. Locomotive Feed In locomotives the feed pumps are made of brass and the plunger of iron or brass. They are worked either from an eye on the back of the eccentric (see fig., p. 70, G), or by the piston crosshead. The passage of the water from the tank to the boiler is governed by three ball valves and a cock or valve box close to the boiler. The lift of the valves must never exceed ~g or of an inch. There are generally two pumps to each engine. The water, when directly admitted to the boilers, enters about the middle of the bottom, but sometimes a pipe passes it through the smoke box first to extract as much heat as it can from the heated gases before it gains admission to the boiler. So also in the marine engine, the water sometimes enters the boiler from round the funnel.
202. (4) Giffard's Injector. – This is a novel contrivance for feeding boilers, fast superseding all other methods of feed; but no convincing explanation of its action has yet been offered. The manufacturers claim for it these advantages : –
(1) It is as cheap as a pump and its connections; (2) it saves the wear and tear of pumps, which in locomotives and other high pressure engines are very considerable; (3) it saves the power required to work the pumps; (4) the water enters the boiler at a high temperature, so no heat is lost; (5) you can feed a boiler without setting the engine in [page 175] motion, thus saving donkey pumps; (6) it is free from risk of damage or stoppage by frost.
We will suppose it properly attached to the boiler, it then works in the following manner : –
GI is the injector, N is attached to the boiler. Steam can pass into the injector at N. When the handle d is moved up, steam rushes through a i at i, where it meets the water supply coming into the injector at E. The steam drives the water through n, and beyond the valve s, into the boiler. When there is sufficient water in the boiler, the valve s is forced upwards, and no more water can pass it; the waste water can then pass through the overflow pipe L. The steam to work the injector must be taken from the highest part of the boiler, and must not be primed. The water driven through it may be taken from a cistern overhead, or from a tank in the ground; but the distance from the level of the water below to E above must not exceed 5 feet. Now it is found that the pressure of steam will actually drive the water into the boiler, although it has to force it against the pressure of both the steam and water in the boiler.
A jet of steam moving with perhaps a velocity of 1700 feet per second, is instantly condensed in perhaps twelve times its weight of water. The combined jet will then move, by the momentum imparted to it by the steam, at one thirteenth its former velocity, 131 feet per second – the motion of the steam being wholly imparted to the water. Thus the jet properly directed enters the boiler, and we can find an explanation of the action of the injector by simply considering that it acts solely by the momentum imparted to the water by the jet of steam.
EXERCISES CHIEFLY FROM EXAMINATION PAPERS.
1. Why is the hydrometer an imperfect instrument without the thermometer (1863)?
2. What quantity of water at 56° E. would be required to condense 1500 cubic feet of steam at a pressure of 35 lbs. per square inch above the atmosphere, so that the temperature of the whole should be 100° E. (1865)?
[page 176] Temperature of injection water is raised 100° - 56° = 44°F. = 24°4/9 C.
100°F. = 37° 7/9C, and temperature at 50 lbs. pressure = 282°F.
Total heat in steam at 282° F. = 1082 + .305 x 282° = 1168° F. = 649°C.
Relative volume at 35 lbs. above the atmosphere, or at 50 lbs., is = 552.
The relative volume may be taken as the number of cubic feet of steam produced from a cubic foot of water.
... Number of feet of water = 1500/532 = 2.71.
The steam has to give up 649 - 37 7/9 = 611°2/9C.
Since each unit gives up 24°4/9 C.
... Injection water required = 611 2/9 / 24 4/9 = 25 times the water evaporated.
... Quantity of water required = 27.1 x 25 = 67.75 cubic feet.
3. When a boiler is filled with sea-water, it is the practice to test the degree of saltness from time to time; why is this? Describe the apparatus employed, and the method of using it (1871).
4. Describe Giffard's injector, and give some explanation of its action (Honours, 1871).
5. Describe the feed pump and valves necessary for supplying the boiler of a locomotive. What is the principle of Giffard's injector (1869)?
6. The brine pump of a boiler being choked, how is the brine to be got rid of, the steam gauge indicating 4 lbs. and the upper surface of the water being 2 feet below the level of the sea (1868)?
Ans. There will be nearly 1 lb. pressure per square inch to clear it.
7. Describe a method of ascertaining the degree of saltness of the water in a marine boiler (1870).
8. How is the degree of saltness of the water in a marine boiler ascertained ?
9. Show generally how to determine the amount of fuel lost by the process of blowing out in marine boilers (Honours, 1871). See questions at the end.
10. Give an analysis of sea water, and state clearly what is the amount of solid matter in it.
11. How is the boiling point of salt water affected by the amount of salt in it ?
12. Describe the manner in which the salt and impurities are "blown off" from the surface.
13. What is How's salinometer? also state the principle on which Seaward's salinometer is constructed.
14. What are the remedies against priming, and what do you mean by priming? can you account for it taking place ?
15. Give the relation between fire-grate surface, heating surface, and the evaporative power of the boiler in a marine engine.
